Examples of our team members' experience
PAT cases

Model maintenance
In a model maintenance study, we help our clients identify the robustness of their predictive models in light of prolonged use. In function of your particular setup, we analyse the applicable boundaries for which your model is valid currently, and will be in the future.
As an example, for spectroscopic models, we evaluate the critical process parameters that could lead to future changes in the model. Further, we try to pinpoint the degrees of freedom where a change in process settings or material properties is unlikely to lead to a decrease in model quality.
Tablet press lead-lag
In-line near infrared spectroscopy inside the feed frame of the tablet press allows to divert tablets that are out-of-specification if the lead-lag between the in-line blend and off-line tablet response is quantified.
High-quality spectroscopic data was collected using a custom-made sampling interface . Subsequently, tanks-in-series models were built to quantify the lead-lag and the key tablet press process parameters affecting the lead-lag were identified. Case studies were performed to demonstrate how this lead-lag should be taken into account during real-time tablet diversion .
In-line near infrared blend potency monitoring can give more confidence about the final tablet quality, reduce the burden of off-line quality testing and potentially improve the process yield.
Process development cases

Twin-screw wet granulator process ranges
With changing raw material properties, pharmaceutical wet granulation requires different process setting values to achieve a good quality product.
Using the historical data collection, in combination with the novel T-PLS modelling approach that brings into account input material properties, blend ratios and process settings at the same time, processability range estimation occurs only based on raw material characterisation.
Experiments only serve to validate or improve the model, reducing the material usage and especially the cost of expensive material input to the minimum.
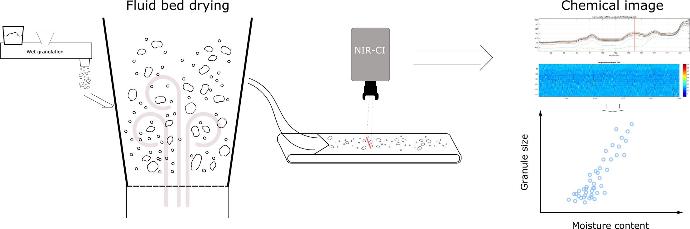
Particle properties
Homogeneity is a key target in pharmaceutical processing, yet one that is labour-intensive to measure.
The capability of hyperspectral imaging was harnessed to overcome this challenge and, for the first time, quantify the chemical composition of individual granules and analyse these in function of granule size and process settings.
Originally developed to assess moisture distributions after fluid bed drying, this technique is currently also used to gain more understanding on the wet granulation process itself and assess fluidisation quality.